Rhodium is a silver-white metallic element that is highly reflective and resistant to corrosion. It is considered the rarest and most valuable precious metal in the world — well above gold or silver. The name rhodium comes from the Greek word “rhodon,” meaning rose, named for the rose-red color of its salts.
Rhodium metal was discovered in 1803 by English chemist William Hyde Wollaston shortly after the palladium discovery. It often occurs with deposits of platinum and is commonly obtained from the mining and refining of platinum.
Rhodium, one of the rarest and most precious metals on Earth, plays a vital role in numerous industries. This platinum group metal, though not as famous as its counterparts such as gold or silver, is an unsung hero powering some of our most crucial technological advancements. With a wide array of applications in automotive, electronics, optics, and more, rhodium’s contribution to our everyday life is undeniable. Let’s delve deeper into understanding why rhodium matters.
Properties of Rhodium Metal
Rhodium is one of the 6 platinum group metals: platinum, palladium, rhodium, osmium, iridium, and ruthenium.
Some common characteristics of the platinum group metals include high melting points, general non-toxicity, and resistance to wear, oxidation, and corrosion, according to Chemistry Libretexts.
What sets rhodium apart are its unique physical and chemical properties. It’s incredibly resistant to corrosion, even more so than other PGMs. It withstands most acids and alkaline solutions and doesn’t tarnish or oxidize easily. This makes it valuable for objects exposed to harsh environmental conditions.
Rhodium metal is also a remarkable catalyst, enabling chemical reactions without being consumed or altered. This property is especially significant in industrial applications.
Additionally, it has a high melting point and low electrical resistance. Its high reflectance of both visible light and heat adds to its array of useful properties.
- It is also classified as a noble metal, meaning that it does not react to oxygen easily, acts as a fantastic catalyst, and is resistant to corrosion and oxidation.
- Rhodium is the rarest of the platinum group, occurring up to 1/200 million in the Earth’s crust
- Has a lower density and a higher melting point than platinum.
- Has low electrical resistance, low and stable contact resistance, and is highly resistant to corrosion.
- Unaffected by air and water up to 1,112 degrees Fahrenheit
- Very hard and has a high reflectance.
- Rhodium has no known biological use and no known use for life processes.
- Although it is generally considered non-toxic, some of its compounds are toxic and carcinogenic. Naturally occurring rhodium consists of just one stable isotope: Rh-103.
Sources: Rhodium metal occurs with other platinum metals in river sands in the Urals and in North and South America. It is found in the copper-nickel sulfide ores of the Sudbury, Ontario region.
Facts & Physical Data about Rhodium Metal
- Atomic number: 45, Atomic symbol: Rh
- Atomic weight: 102.90550, Atomic Radius (pm): 134, Atomic Volume (cc/mol): 8.3, Density: 12.41 grams per cubic cm
- Phase at room temperature: Solid
- Melting point: 3,567 F (1,964C), Boiling point: 6,683F (3,695C)
- Period number: 5, Group number: 9
- Electron configuration: [Kr] 4d85s1
- Number of isotopes (atoms of the same element with a different number of neutrons): 24 whose half-lives are known; one stable; Most common isotopes: One stable isotope Rh-103
- Covalent Radius (pm): 125, Ionic Radius: 68 (+3e)
- Oxidation States: 5, 4, 3, 2, 1, 0
- Lattice Structure: Face-Centered Cubic
Did you Know?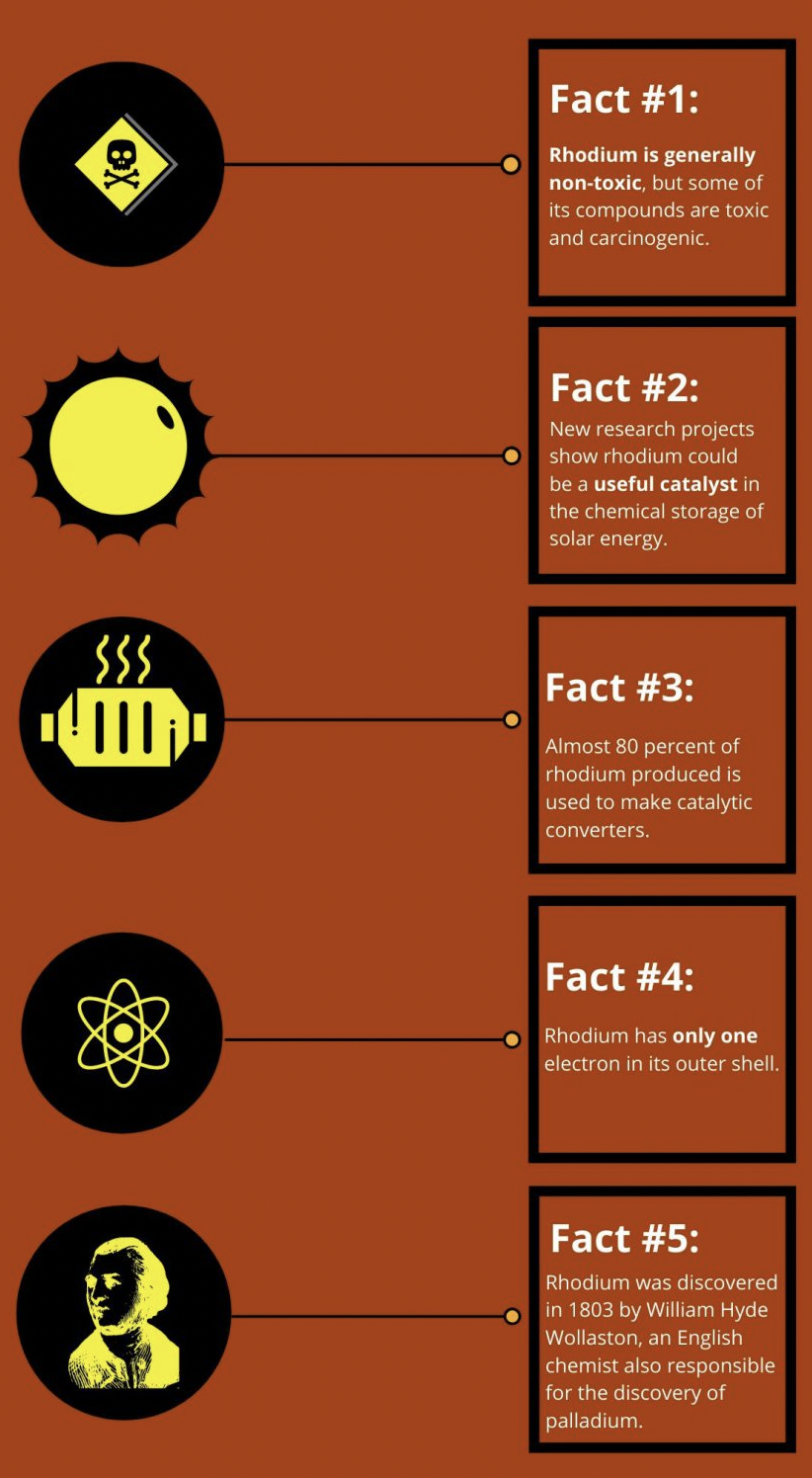
- An alloy of rhodium-platinum is used in heart pacemakers.
- South African PGM producers extract a mix of metals comprising approximately: 60% platinum, 30% palladium, and 10% rhodium.
- Resistant to most acids.
- Rarely used by itself and almost always as an alloy.
- Out of 3 precious metals (rhodium, platinum, and palladium) used in vehicle catalytic converters, rhodium has by far the highest activity for the removal of nitrogen oxides (NOx) from the exhaust.
- Has very high activity for the oxidation of hydrocarbons (HC) and carbon monoxide (CO)
- Has very good resistance to the poisons present in the exhaust stream
- Its primary drawback, however, is its high cost.
- All rhodium compounds are easily reduced or decomposed through heating to create powdered (or sponge) metal.
Use of Rhodium Metal
- Due to its low electrical resistance and its high corrosion resistance, it is used as an electrical contact material as well, according to RSC.
- Since rhodium is quite brilliant and resistant to tarnishing, it is used as a finish for jewelry, searchlights, and mirrors.
- Plated rhodium is very hard and has a high reflectance, which makes it useful for optical instruments and jewelry.
- Rhodium is also used as a catalyst in certain reactions.
- Other uses for rhodium are coating optic fibers, crucibles, thermocouple elements, and headlight reflectors.
Rhodium Metal Alloys
Rhodium is often alloyed with platinum and iridium to make an oxidation-resistant metal that can stand against high temperatures. One major use of rhodium is as an alloying agent to harden platinum and palladium.
It is also alloyed with platinum for aircraft turbine engines.
These alloys are used in furnace windings, pen nibs, phonograph needles, high-temperature thermocouple and resistance wires, electrodes for aircraft spark plugs, bearings, and electrical contacts.
Rhodium as Catalytic converters
The main use for rhodium is in catalytic converters designed to clean vehicle emissions. Rhodium — often together with palladium and/or platinum — accomplishes this by reducing nitrogen oxide in the exhaust gas.
Without rhodium catalysts, the air in our cities would be much worse due to vehicle exhausts.
In the chemical industry, rhodium is used as a catalyst in the making of nitric acid, acetic acid, and hydrogenation reactions, according to the Royal Society of Chemistry (RSC).
Rhodium in the Automotive Industry
The automotive industry is the primary consumer of rhodium, utilizing its excellent catalytic properties to control vehicle emissions. Rhodium-based catalysts are a critical component of three-way catalytic converters in vehicles.
Catalytic converters transform harmful gases emitted from vehicle exhausts into less harmful substances. Rhodium acts as a catalyst for reducing nitrogen oxides, a significant contributor to air pollution and smog. As regulations on vehicle emissions become stricter worldwide, the demand for rhodium is likely to surge.
Rhodium also finds potential use in hydrogen fuel cells, an emerging clean energy technology. Rhodium-based catalysts can enhance the efficiency of hydrogen-oxygen reactions, thereby contributing to the growth of sustainable transportation.
Rhodium in the Electronics Industry
In the electronics industry, rhodium’s excellent corrosion resistance and low electrical resistance come into play. It’s used for plating electrical connectors, contacts, and switches, ensuring their reliability and durability. This is critical for devices operating in high-wear, high-temperature, or harsh environmental conditions.
Rhodium’s Role in Optics and Surface Science
Rhodium’s high reflectance of light and heat is beneficial in the field of optics and surface science. High-quality mirrors, especially those used in powerful telescopes, are often coated with a thin layer of rhodium. This allows astronomers to gather and analyze data from distant stars and galaxies more effectively.
Similarly, rhodium is used to coating the interior of high-performance headlamps and searchlights. Also helps improving the efficiency and brightness of these lighting instruments.
Furthermore, rhodium is useful in X-ray monochromators, which isolate specific X-ray wavelengths from a broader spectrum. The rhodium-coated surface enhances performance, providing more accurate results.
Investment Potential of Rhodium
Rhodium also holds immense value as an investment asset. Due to its rarity and high demand, rhodium often commands a higher price per ounce than other precious metals. While the price of rhodium can be quite volatile, strategic investment in it has yielded substantial returns for some investors in the past.
However, investing in rhodium is not without risks. The market for rhodium is relatively small and illiquid compared to other precious metals like gold or silver. This, coupled with its high price volatility, makes it a speculative investment that requires careful consideration.
Rhodium and Sustainability
As the world pivots towards a more sustainable future, rhodium’s role is growing. Its catalytic properties are integral to reducing harmful emissions and pollution. Moreover, its potential application in clean energy technologies like hydrogen fuel cells aligns with global efforts to transition towards greener, sustainable energy sources.
Moreover, the recycling of rhodium, primarily from catalytic converters, has become an important source of this metal. This not only helps meet the increasing demand but also promotes a circular economy. This is useful for recycling as long as possible.
BONUS: Rhodium Metal Jobs
Rhodium’s versatile applications across various industries create an array of job opportunities. Here are a few examples:
- Automotive Engineers: They work with rhodium in developing and improving catalytic converters, focusing on making vehicles more environmentally friendly by reducing emissions.
- Chemical Engineers: They leverage rhodium’s exceptional catalytic properties in various industrial chemical reactions, working to optimize efficiency and safety.
- Electrical Engineers: They use rhodium in electronics for its high corrosion resistance and low electrical resistance, particularly in the manufacturing of durable connectors, switches, and contacts.
- Metallurgists: Involving in mining and refining rhodium, as well as in developing new alloys and applications for the metal.
- Material Scientists: They research and develop new materials using rhodium, focusing on its unique properties such as high reflectance and resistance to corrosion.
- Investment Analysts: They monitor and analyze the rhodium market, providing investment advice to clients interested in precious metals.
- Recycling Specialists: They focus on extracting rhodium from discarded catalytic converters and other waste products, contributing to the circular economy and sustainable practices.
- Quality Control Inspectors: They ensure the quality and safety of rhodium and its products, adhering to industry standards and regulations.
Each of these roles plays a part in the journey of rhodium, from its extraction and processing to its application and eventual recycling. There is growing importance of rhodium in various industries. Job opportunities is going to rise up.
Conclusion
Rhodium may not share the limelight with more popular precious metals, but its contribution to technology and sustainability is indispensable. From making our cars cleaner to facilitating deep space exploration, rhodium plays a part in driving our world forward. Its unique properties, combined with its myriad applications in diverse fields, make it an element that truly matters.
The importance of this precious metal has promising growth. Rhodium, indeed, matters and it’s time we start recognizing its value.





